To treat
posterior segment eye diseases, four routes are available i.e., topical,
periocular, intraocular and systemic. Topical and systemic routes are not
preferred route of drug administration because of having some significant disadvantages
i.e., low ocular bioavailability of drug, frequent administration of high
amount of drugs1.
Periocular route is the most preferable
route for instillation of drug to the posterior segment of eye2
ensuring higher retinal and vitreal drug bioavailability (0.01-0.1%) which is
higher than topical medication
(≤ 0.001%)3. Sub-tenon route is most preferable periocular
route. The disadvantages of this route include cataract, hyphema, corneal
decompensation and rise in intra ocular pressure. Disadvantages of intravitreal
steroid injections are worse than periocular sub-tenon pathway. Therefore,
sub-tenon route is preferred pathway for administration of steroid4.
Intravitreal injections have earned fame
among researchers and clinicians. Unlike topical and systemic routes, it offers
high concentration of drug to vitreous, retina and choroid5. Though
it ensures bioavailability of drug, instillation of drug through this pathway
is invasive and potentially risky which causes endophthalmitis, retinal
detachment and vitreous hemorrhages6.
Scientists have made many efforts to
enhance bioavailability of drug by designing different drug delivery systems
i.e. ointments, suspensions, gels, collagen shields, implants and hydrogels7.
In general there are three types of implants available in market for treatment
of posterior segment eye diseases: non-biodegradable, biodegradable and stimuli
responsive implants. In non-biodegradable implants, FDA has approved Vitrasert®
and Retisert®. Former implant carries ganciclovir drug for treatment
of Cytomegalo virus retinitis. It releases drug for 8 months. While the later
implant carries fluocinolone acetonide to treat chronic non-infectious
posterior uveitis. Iluvien® implant is waiting for FDA approval but
accepted in some EU countries. For degradable implants, FDA has approved only
Ozurdex®. None of these implants are available in Pakistan for
treatment of patients suffering from posterior segment eye diseases. However, each system has its own advantages
and disadvantages.8
Among all such devices, in-situ forming hydrogel
has gained enormous attention by scientists. These hydrogels are liquid at room
temperature and solid under physiological conditions9. These in-situ
hydrogels can be achieved by several ways such as pH change, ionic cross
linkage and temperature modulation. Among all these, thermosensitive hydrogels
got immense attention for ocular treatments because of its easy handling and
low viscosity at room temperature10,11.
Chitosan,
(poly-β(1,4)-d-glucosamine),
has been extensively used as implant in the form of gels, fibers and membranes
in the field of tissue engineering and biomedical sciences and drug controlled
release systems. Since chitosan is highly biocompatible, therefore, it has been
extensively used for the synthesis of thermosensitive hydrogels which help to
treat ocular diseases. Various drugs have been loaded on to chitosan based
thermosensitive hydrogels for treatment of ocular diseases for example, latanoprost
was loaded on chitosan and gelatin based thermosensitive hydrogel for
controlling ocular hypertension12. Chitosan in combination
with disodium α-d-glucose 1-phosphate (DGP) has been used for ocular drug
delivery system13. A novel copolymer, poly (N-isopropylacrylamide)–chitosan
(PNIPAAm–CS), was investigated for its thermosensitive in situ gel-forming
properties and potential utilization for ocular drug delivery14.
Another novel thermosensitive hydrogel was made by using chitosan and
glycidyltrimethylammonium chloride (GTMAC) and named as N-[(2-hydroxy-3-trimethylammonium)
propyl] chitosan chloride (HTCC)15.
Chitosan has been used
as a carrier of dexamethasone drug to treat ocular diseases i.e., Mucoadhesive chitosan-coated cationic microemulsion of
dexamethasone for ocular delivery16,18. Keeping in mind all such
information, in present work, we are for the first time, aiming to use
biodegradable chitosan thermosensitive gel loaded with dexamethasone and
finding the potential use of sub-tenon space for insertion of these synthesized
gels. This will provide sustained release of drug and will overcome the side
effects of previous treatments to treat posterior segment eye diseases especially
macular edema and uveitis. The cost of pre-existing treatments of these
diseases are expensive and sometimes unaffordable when considering needs of
individual patient.
MATERIAL AND METHODS
Chitosan (DD = 80.91% and Mol. Wt. =
25992.88) was synthesized in our laboratories. Acetic acid (CH3COOH)
was purchased from Riedel-deHaen (origin). From Bio world (origin) PBS
(Phosphate Buffer Saline) was bought. NaHCO3 was bought from Daejung
chemicals and metals CO., LTD (Korea). Dexamethasone was bought from Zhejiang
Xianju Junye pharmaceutical Co., Ltd (China). NaCl was obtained from Omicron
sciences LTD (UK). From Sigma-Aldrich (Germany) sodium hydroxide (NaOH) was
bought.
In acetic acid (0.5 M, 2.5 ml) Chitosan
(0.2g) was dissolved and stirred for 1 hour and 30 min at room temperature.
Powdered dexamethasone (3.5 mg) was added and stirred for further 30 min at
room temperature. This solution was placed at 4°C for 30 minutes to cool
it down. After this, NaHCO3 solution (0.48 M, 2 ml) was added drop
wise. In the meanwhile, pH change was monitored and finally maintained at 7.
Constant stirring was done to remove effervescence. Once required pH was
achieved, solution was placed inside oven at 37°C. Formation of gel started
after 3-5 minutes and it happened from the surface first. It took 2 hour for complete
conversion of liquid into hydrogel.
Test tube invert method was used to analyze
sol-to-gel transition. In this method, 0.5 ml polymer solution of given
concentration was taken in 3 different vials. The vials containing polymer
solution were placed at 4°C for 30 min – 1 hour. After this, each vial was
immersed in separate water bath having different temperatures i.e. 10°, 25° and
37°, for 10 min. After 10 mins, each vial was taken out and inverted to
180°. If no visible flow was observed
within 30s of inversion, sample was considered as “gel”.
The pH of sample before and after gelation
was calculated by calibrated pH meter (Eutech instrument pc 150). Neutral pH is
the indication of completion of reaction between acid and base which is
required for conversion of sol-to-gel. Also, acidic or basic gel implant can
cause irritation to the eye and can permanently damage the tissue. Therefore,
it is important to measure the pH of the gel.
Structural characterization of prepared
thermosensitive hydrogels was carried out by Fourier transfer infrared (FTIR)
spectroscopy, coupled with smart ATR accessory. Thermo-Nicolet 6700P FTIR
Spectrometer (USA) was used and the average number of scans were 256 at the
resolution of 8 cm-1. Spectra that were recorded ranged in
wavelength of 4000-650cm1.
Scanning electron microscope (Tescan, Vega
LMU) at 10 kV under low vacuum mode at 10 Pa was employed for the assessment of
pore size and compact structure of synthesized hydrogel. At different
magnifications images were obtained. Image processing software (Image J) was
used to calculate the diameter of pore by selecting 30 pores randomly.
For every
sample composition (n=3) degradation tests were performed gravimetrically. Two
weights were taken to achieve this purpose. Before immersing them into
solutions, initial dry weight (![]() ) of hydrogels was taken. Then
the samples were kept in phosphate buffered saline (PBS), lysozyme solution
(1mg/ml) in PBS at 37oC for different time points (day 1-day 28).
The samples were taken out at each time point, dried at 37oC for 24
H and subsequently weighed (
) of hydrogels was taken. Then
the samples were kept in phosphate buffered saline (PBS), lysozyme solution
(1mg/ml) in PBS at 37oC for different time points (day 1-day 28).
The samples were taken out at each time point, dried at 37oC for 24
H and subsequently weighed (![]() ). The dried weight (without
water content) remaining ratios were determined as following:
). The dried weight (without
water content) remaining ratios were determined as following:
![]()
Drug
release test was carried out in PBS. For this purpose, powdered dexamethasone
was added in stirring solution of chitosan and sodium bicarbonate wherein drug
content was 0.7 mg/ml. After adding drug, resultant mixture was placed in oven
at 37°C to form gel. Hydrogel was cut into triplicates of equal weight (10 mg)
and dipped into 5 ml PBS solution. At different time intervals (after 3 h,
after 16 h, after 24 h and after 48 h) PBS solution in vials was replaced with
the fresh one. Collected PBS solutions were analyzed under UV/Visible
spectrophotometer (Perkin Elmer). Amount of drug release was determined by
following straight line equation:
![]()
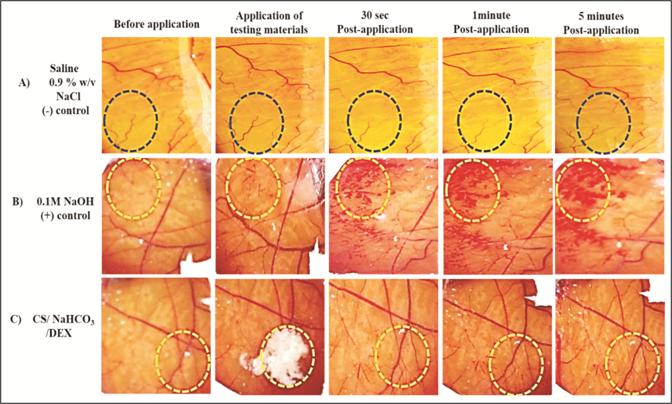
HET-CAM (Hen’s egg test –
chorioallantoic membrane) assay, most robust and successful assay, was used to
evaluate irritation properties of chemicals and consumer products that might come
in contact with human eyes. The assay covers a broad spectrum of chemicals with
whole range of degrees of irritation and physical appearances of different
substances. To evaluate the ocular tolerance of the developed thermosensitive
hydrogel, HET-CAM test was performed with small modifications.
Briefly, freshly
fertilized hen’s eggs were bought from Big Bird Group (Lahore, Pakistan). They
were put in an incubator at 37.8 ± 0.5°C and 55% humidity for nine days. At day 10, the egg shell
was opened, and white egg membrane was removed carefully without injuring any
underlying blood vessels. Subsequently, the surface of the CAM was exposed to
0.1g of the test substance, 0.1 M sodium hydroxide (NaOH) solution (positive
control), and a 0.9% NaCl w/v saline solution (negative control). The chorioallantoic membrane and
its clearly delineated vascular system was further assessed subjectively in
terms of hyperemia, hemorrhage
or coagulation. Changes were examined using a light microscope (Mitotic, China)
before exposure and at different time points post-application for 5 min.
Scoring of each test substance was designated by using a classification
system previously described by Luepke
and Kemper (1986): Non
irritation: up to 0.9; slight
irritant: 1-4.9; moderate irritant:
5-8.9; severe irritant: 9 and
above. Moreover, images were obtained before application and for 30 s, 2 min,
and 5 min after exposure.
RESULTS
In the preparation
of Thermosensitive Hydrogel of Chitosan and loading with Dexamethasone, it was
shown that neutralization occurred which resulted in the formation of
physical junctions (hydrogen bonding) between polymeric chains of chitosan. In chemical structure analysis by Fourier
Transform Infrared Spectroscopy it was shown that

Fig. 1: Step by step illustration of
synthesis of thermosensitive hydrogel.
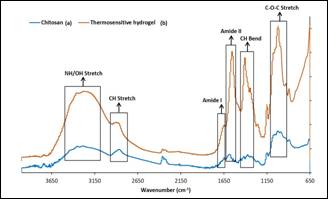
Fig. 2: FTIR results of pure
chitosan (a) and thermosensitive hydrogel (b).
only temperature changed
the physical appearance of polymer which was not significant.
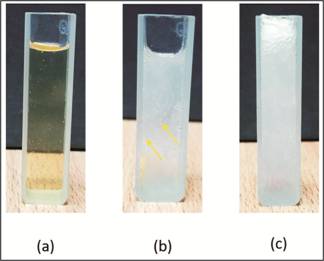
Fig. 3: Sequence of pictures illustrating
physical changes occurred at 37°C. (a) Clear solution mixture before gelation.
(b) Gelation started at 37°C. (c) Gelation completed at 37°C. Yellow arrows are
indicating effervescence of CO2.
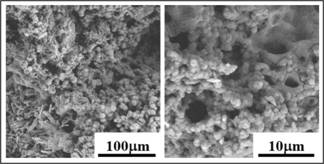
Fig. 4: Scanning electron
micrographs of synthesized hydrogel (magnification bars are given with each
image).
Characterization
of Sol-to-Gel transition temperature by Test-Tube invert method showed that no CO2 was released at 4°C hence no gelation occurred.
At 25°C, no gelation was observed within 10 minutes. But after immersing vial
for 2 h, gelation started at very slow rate. Best results were obtained at
37°C. Before gelation the pH was 4.9 and after gelation it was 7.14.
In-vitro drug
release results showed that hydrogel can stay in sub-tenon region of eye
over a month and release drug. Based on these results, we are proposing that
our synthesized biomaterial will be the first thermosensitive chitosan based
hydrogel which will support sustained release of dexamethasone in sub-tenon
region of the eye.
To assess degradation potential of synthesized biomaterial,
in vitro degradation test were performed.
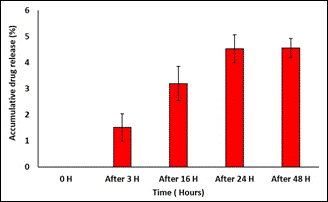
Fig. 5: In vitro accumulative
release of dexamethasone from chitosan hydrogel.
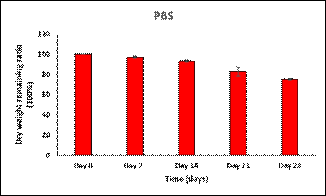
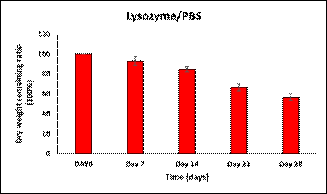
Fig. 6: In vitro degradation in
PBS and Lysozyme.
We managed to mimic the physiological
environment by selecting two media for degradation; PBS and lysozyme. From the
results, it was concluded that synthesized materials were degradable. PBS and
lysozyme, both caused degradation to synthesized hydrogels. Statistical
analysis revealed significant difference (p = 0.0005) between the degradation
values of day 7 and 28.
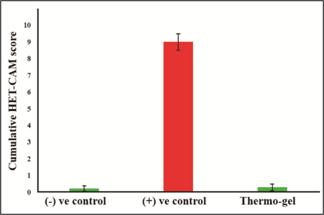
In ocular irritancy test by HET-CAM
which is a semi qualitative test to asses the irritation potential of a testing
material. From figure 8, it was concluded that synthesized thermosensitive
hydrogel was not irritant.
DISCUSSION
In the
preparation of Thermosensitive Hydrogel of Chitosan and loading with
Dexamethasone, the gelling mechanism of solution mixture of chitosan at 37°C involves neutralization of chitosan solution in the presence
of NaHCO3 (Figure 1). When chitosan is dissolved in 0.5M acetic acid
solution, protonation of amino groups of chitosan takes place. At this point
the pH of solution is 4.9. As 0.48M of NaHCO3 solution is added into
the 0.5M of chitosan solution, CO2 evolves. By experimentation, it
is concluded that this neutralization reaction occurs only at or above 37°C19.
In
chemical structure analysis by Fourier
Transform Infrared Spectroscopy, FTIR spectra are obtained for pure powdered
chitosan and synthesized thermosensitive hydrogel. Broad peak between 3200-3500
cm-1 appears due to NH/OH stretching vibrations. The absorptions
present in the range of 2919-2910 cm-1 are assigned to CH stretching
vibrations and peaks for CH bending vibration were present around 1400 cm-1,. The absorptions around 1650 and 1585 cm-1 are
attributed to amide I (-C = O stretch) and amide II (-C-N stretch and -C-N-H
bending vibrations), respectively20. It is found that C-O-C deformation band appears around 1097cm1
Characterization of Sol-to-Gel transition temperature by Test tube invert
method is used to analyze the temperature required for sol-to-gel transition.
For this purpose, 5 mL of fresh chitosan/NaHCO3 mixture having
dispersed dexamethasone is added into vial. This vial is immersed in water bath
for 10 minutes at three different temperatures: 4°C, 25°C and 37°C. Gelation
time is observed by tilting vial at an angle of 90° for 1 min till no flow.
From
results it is observed that no CO2 is released at 4°C hence no
gelation occurs. At 25°C, no gelation is observed within 10 minutes. But after
immersing